
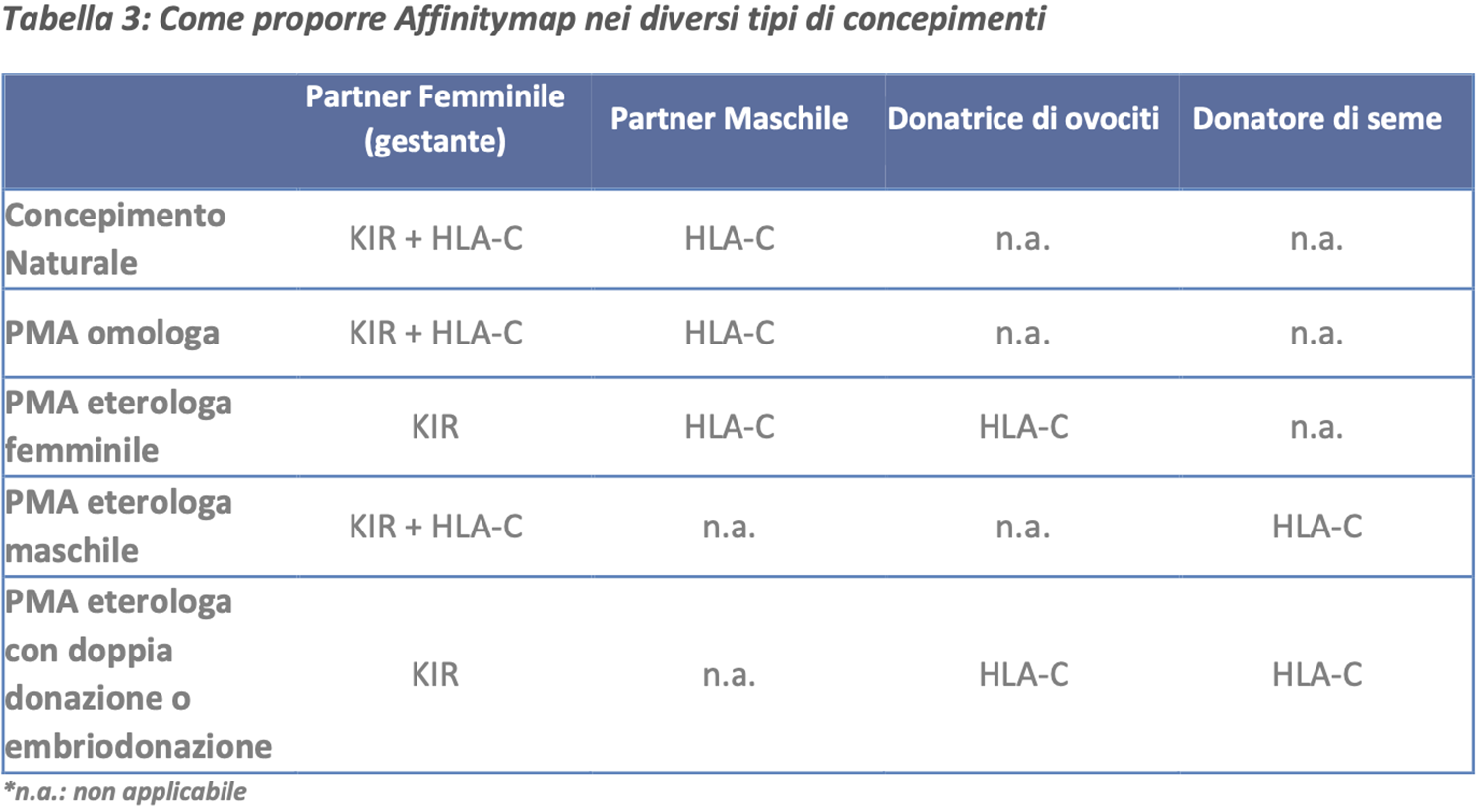
Affinitymap is a test for the genotyping of KIR and HLA-C genes aimed at assessing the compatibility between KIR receptors, present on maternal NK lymphocytes in the uterine cavity, and the fetal-origin HLA-C histocompatibility system.
During pregnancy, the maternal immune system develops a 'tolerance' to the embryo first and then to the fetus, despite recognizing it as partially non-self. The success of the pregnancy is therefore safeguarded by the ability of the maternal immune system, through KIR receptors, to tolerate the embryo and not attack it as a foreign element.
In the presence of optimal compatibility, the maternal-fetal tolerance process will develop correctly, facilitating a pregnancy without complications; conversely, an incompatibility between maternal KIR genes and fetal HLA-C will lead to the activation of the maternal NK population, resulting in an immune response that could compromise the success of the pregnancy or lead to preterm birth.
AFFINITYMAP
Available panels
Affinitymap KIR: KIR genotype analysis
Affinitymap HLA-C: HLA-C genotype analysis
Affinitymap HLA-C/KIR: KIR genotype analysis and HLA-C genotype analysis
AFFINITYMAP
Who it's recommended for
Affinitymap is recommended in all cases where it is necessary to investigate maternal-fetal immunological compatibility regarding the KIR/HLA-C genotype for the proper management of the couple in the ART process or during the preconception phase.
It's particularly recommended for:

Couples with a clinical history of recurrent miscarriages and/or repeated implantation failures

Couples with a history or risk of pre-eclampsia

Selection of sperm donors in male heterologous assisted reproduction

Selection of egg donors in female heterologous assisted reproduction

Selection of donors in the case of embryo donation/double gamete donation
AFFINITYMAP
Analysis method
Affinitymap allows for the detection and genotyping of the following allelic series: HLA-C*01, HLA-C*02, HLA-C*03, HLA-C*04, HLA-C*05, HLA-C*06, HLA-C*07, HLA-C*08, HLA-C*12, HLA-C*14, HLA-C*15, HLA-C*16, HLA-C*17 and HLA-C*18.
The test involves the extraction of DNA from peripheral blood, followed by amplification of the HLA-C genetic markers through PCR and subsequent typing using specific SSO (Sequence Specific Oligonucleotide) probes linked to Luminex microspheres (Lifecodes, Inmucor).
The test involves the extraction of DNA from peripheral blood, followed by amplification of genetic markers associated with KIR genes through PCR and subsequent typing using SSO (Sequence Specific Oligonucleotide) probes linked to Luminex microspheres (Lifecodes, Inmucor).
This technique allows for the detection and genotyping of 16 KIR genes: KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL4, KIR2DL5, KIR3DL1, KIR3DL2, KIR3DL3, KIR3DS1, KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5, KIR2DP1 and KIR3DP1.
Scientific publications in support
(1) Marsh SGE et al. Killer-cell Immunoglobulin-like Receptors (KIR) Nomenclature Report, 2002; Human Immunology, 2003 64:648-654.
(2) Hong Y et al. Killer immunoglobulin-like receptor repertoire on uterine natural killer cell subsets in women with recurrent spontaneous abortions. Eur J Obstet Gynecol Reprod Biol. 2008 Oct;140(2):218-23.
(3) Hiby SE et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success, J Exp Med 2004; 200: 957-65.
(4) Hiby SE et al. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage, Hum Repro, 2008; 23: 972-6.


